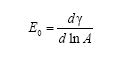|
November 2008 |
||||||||||||||||||||||
| Surface Dilatational Elasticity and Viscosity | ||||||||||||||||||||||
|
The axisymmetric bubble shape method has
proven useful in the measurement of surface dilatational elasticities
and viscosities.1 In the referenced study dynamic surface
tension as well surface dilatational viscoelastic properties are
measured by way of the oscillating bubble. A ramé-hart goniometer with
our software-controlled Automated Dispensing System, Environmental
Chamber, Temperature Controller, and DROPimage Advanced software in
conjunction with a specially-designed oscillating unit were employed to
take the measurements. The schematic below illustrates the layout of
equipment. Read the next section to learn more about our new Oscillator
which is used in the study of surface dilatational rheology.
A surfactant (surface acting agent) is generally an agent used to promote wetting by lowering surface tension through adsorption. Surfactants can also reduce interfacial tension between two liquids -- such as oil and water. Polymer surfactants, as observed by Hansen, et al, produce higher surface elasticities than simple surfactants. In their study, cationic polysoaps were synthesized and evaluated. It was found that both the monomers and polymers studied exhibited strong surface activity. Fluorinated compounds showed lower surface tensions and much higher surface dilatational elasticity than their hydrocarbon counterparts. Adsorption is slower, too. It's not possible to fully explain adsorption and surface tension of surfactants, emulsions, and foaming agents using static methods. When a surface is expanded and contracted, the surface tension has been traditionally explained using Gibb's formula:
where γ is is the surface tension and A is surface area. This term does not allow for time-dependant responses. Thus a more replete equation is now used to define complex surface elasticity:
where E' is the storage modulus and E'' is the loss modulus. The measurement of elasticity for many surfactants can be best accomplished through the use of periodic oscillatory deformation. The oscillating bubble was first introduced by Lunkenheimer2 who used Laplace pressure to measure the surface tension. The updated method uses a sessile or pendant drop or bubble and DROPimage software to measure the surface tension, drop volume, and surface area using axisymmetric shape analysis and by creating oscillatory drop deformations the dynamic properties can be measured. The method works best with an oscillation frequency under 2-3 Hz. At higher frequencies, super-harmonic effects can distort measurements. The oscillation method has been shown to be an effective tool for the measurement of surface dilatational elasticities and to better understand dynamic surface and interfacial tension. 1 Fromyr, T.; Hansen, F. K.;
Kotzev, A.; Laschewsky, A.; Adsorption and Surface Elastic Properties of
Corresponding Fluorinated and Nonfluorinated Cationic Polymer Films
Measured by Drop Shape Analysis, Langmuir 2001, 17, 5256-5264 |
||||||||||||||||||||||
| New Product Announcement - Oscillator | ||||||||||||||||||||||
|
This month we are pleased to announce our new Oscillator, p/n 100-28, which complements our Automated Dispensing System and is optimized for studying surface dilatational rheology as detailed in the above section.
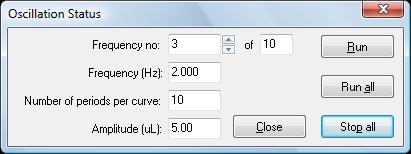
This tool is fully software-driven and supported by DROPimage Advanced v2.4 or above. It requires our software-controlled Automated Dispensing System (p/n 100-22-100) and current-generation Model 250-F1 or 500-F1 Goniometer or a legacy model which has been sufficiently upgraded. The Oscillator sits inline between the dispenser and the dispensing tip as detailed in the schematic in the section above. A serial interface provides communication between the PC and the Oscillator. DROPimage provides a dialog box (see below) allowing the user to manually control the device.
But perhaps the most powerful way to use the Oscillator is to integrate oscillation control into the experiment design using the methods editor. With this approach, the oscillation can be fully integrated into an experiment. Additional parameters can be set to control the drop volume, timing, and events. The dialog appears at the start of running a previously defined method.
After the experiment is run, the results window (shown below) reports an array of data including:
Note that E' and E'' can also be plotted as a function of frequency. DROPimage has been upgraded to fully support the Oscillator. On the rear of the Oscillator, there is a control knob which allows the user to manually move through a complete cycle in order to start at the precise step in the cycle desired. On the front of the unit, there is a peep hole which allows access to the stroke adjustment. The stroke volume can be set to between 0 and 250µl, but most experiments will use stroke volumes under 10µl. The periodic oscillation frequency can be set anywhere between 0 and 25Hz (but is typically under 5Hz) and is controlled in DROPimage via the method editor or the Oscillator Control dialog box.
For more information on
the Oscillator including a copy of our PDF brochure for this product, or for a quotation, please contact
us. On request, we can also send more detailed and technical information on
surface dilatational rheology theory and how our DROPimage Advanced
software employs it. |
||||||||||||||||||||||
|
||||||||||||||||||||||