|
|
|
|
July
2016 |
|
| Superhydrophilicity and Pinning | |
|
We have covered topics relating to
superhydrophobicity many times in this newsletter. However, we speak
about superhydrophilicity with much less frequency. Perhaps this is
mostly due to the inordinate amount of research focused on the
fabrication of nano-textured surfaces optimized for nearly non-wetting
behavior. Much less research is focused on the making surfaces that
exhibit complete wetting. Or perhaps it's the result of a lack of
consensus on what defines a superhydrophilic surface. More on that in a
minute.
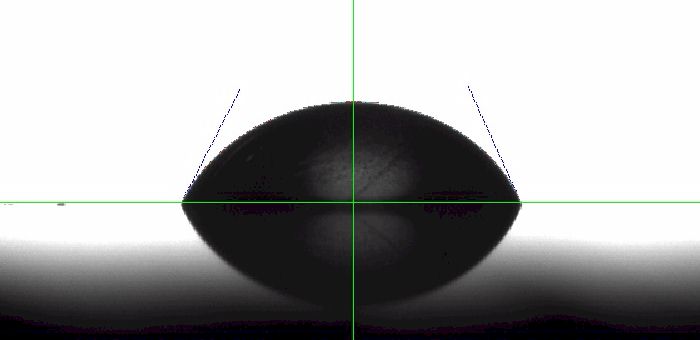
Solid surfaces are often corralled into two groups: low and high surface energy solids.1 The low-energy solids, such as polymers, exhibit weak van der Waals bonds and the resulting behavior is poor wetting. The water contact angle on PTFE, for example, is typically over 100° depending on roughness. High-energy solids, such as metals and ceramics, produce lower contact angles as a result of the stronger bonds that hold them together. This translates to lower contact angles. You may expect a high-energy solid surface that has been polished and cleaned to have a water contact angle approaching 0°. However, that is often not the case. To test this behavior, we took a piece of polished 6061 Aluminum from our shop (not anodized) and measured a static contact angle of 65.6°. See image Figure 1 below.
There seems to be some debate as to the reason for this unexpected behavior. Some earlier researchers2 proposed that it's due to organic contaminants on the surface. However, others suggest strong pinning behavior. To explore this behavior, we allowed the drop to evaporate over a twenty minute period. If you recall from last month's discussion, evaporation is one way to measure the receding contact angle. Today we're more interested in what happens as the drop evaporates.
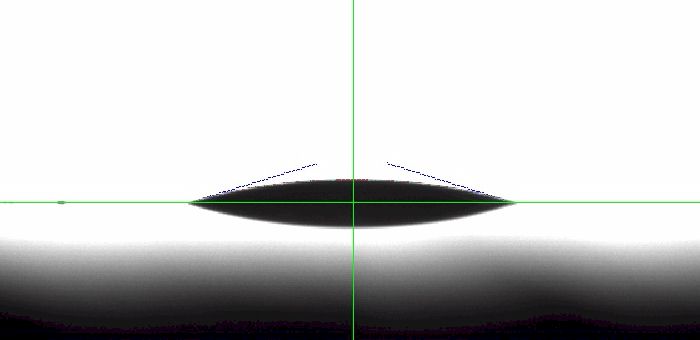
On the polished aluminum surface, the drop width did not decrease over that time indicating that the three-phase line remained pinned while evaporation occurred. The contact angle dropped to 17.2°. See Figure 2 above. The difference between the initial water contact angle and the contact angle after 20 minutes of evaporation translates to 48.4° and indicates a large contact angle hysteresis.
We continued to allow the drop to evaporate and finally the three-phase line began to contract. In Figure 3 above the water contact angle measures 7.3° while the three-phase line has contracted 16.9%. Depinning only occurs when the contact angle is extremely low - under about 10° in this case. When we measure an evaporating water drop on a polymer surface, by contrast, the behavior is quite a bit different. To illustrate this, we took a cleaned PTFE surface and measured the dynamic contact angle. Figure 4 shows the change in drop size (red) and the change in contact angle and three-phase line (in blue). Note that throughout the experiment, the left side remained pinned while the right side contracted. This may be due to a contaminant on the surface, a nick or other change in topology from one spot to another, or a small difference in chemical composition.
The graph in Figure 5 shows that for the first 1000 seconds, the drop remained pinned (no change in width) as the contact angle dropped from 105° to 85°. At that point the three-phase line began contracting while the contact angle remained fairly stable at about 80° for the next 1000 or so seconds. If you look closely, you can see (3) different stick-slip events - one at about 1100 seconds, another at 1250 seconds and a third at about 1600 seconds. In these three events the three-phase line contracted abruptly and the contact angle spiked momentarily. With some polymer surfaces, this stick-slip phenomenon is more prevalent.
It's suggested that a weak interaction between the water drop and the polymer surface explains this weak pinning behavior. Furthermore, it's also suggested that we think of surfaces in terms of being strongly pinning (e.g., aluminum and other metals) and weakly pinning (e.g., PTFE and other polymers).3 Bullets
1 See
https://en.wikipedia.org/wiki/Wetting#High-energy_vs._low-energy_surfaces.
|
|
| Super Summer Coupons | |
|
For the month of July, we are running a promotion for a free Automated Dispensing System with the purchase of a Model 500 Advanced Goniometer / Tensiometer. Or get 50% off the cost of an Automated Dispensing System with the purchase of a new Model 200, 250, 260 or 400. For product details on the Automated Dispensing System, see: http://www.ramehart.com/dispenser.htm. For product details on our line of goniometer and goniometer/ tensiometer instruments, see: http://www.ramehart.com/goniometer.htm. To receive a quotation with this promotion, please contact us and let us know which model you are interested in.
For the months of July and August, we are offering $35 off any prebuilt coaxial needle. This coupon is available online only. To place an order, go to http://www.ramehart.us/prebuilt-coaxial-needle/, select the desired size and option and then add to cart and checkout. During checkout, simply use coupon code PREBUILT35. To learn more about our coaxial and triaxial needles, please visit our new website: http://www.customspinnerets.com/. |
|
|
|
Regards,
Carl Clegg |