|
April 2010 |
||
| What is a Contact Angle? | ||
|
Today we will address a few basic questions:
What is a contact angle? What is surface energy? What is surface
tension? And how do these metrics explain wetting, adhesion, and relate
to each other? In a common system used to measure contact angle, a liquid sessile drop rests on a solid flat homogenous surface. The lack of complete wetting results in a finite surface area shared by the drop and the solid. At the point where the drop and solid and external gas phase meet, also known as the three-phase contact line, an imaginary line coplanar with the profile view of the drop can be formed which passes through the three-phase contact line and continues tangent to the drop profile. We'll call this the contact line. A second line, called the baseline, is formed by passing a line through the two points on that three-phase contact line as viewed in the same profile; the baseline passes through the liquid/solid interface. The angle formed between the contact line and the baseline is the contact angle and is commonly represented by θ as shown in the graphic below. Young's equation is used to describe the adhesive and cohesive forces that affect contact angle and which are explained in fuller detail below. Contact angle plays an important role in characterizing materials and surface conditions as well as the effect of surface treatments and contamination.
Consider for a minute the structure of the solid at an atomic level. There are many atoms which bond together naturally with their nearby neighbors and are electrically neutral. However, at the very outer surface of the solid there are atoms which have fewer neighbors with which to bond resulting in a number of unsatisfied bonds. These unsatisfied bonds on the solid's surface result in the solid surface free energy. When matter (such as a liquid drop) is brought next to this surface, the unsatisfied bonds seek to bond resulting in an apparent force. This adhesive force will result in wetting, or spreading, of the liquid. Surface energy is a property of the solid. Now let's focus on the liquid drop. In a manner similar to the solid, the liquid is made up of atoms and molecules which bond to one another as shown in the graphic below. However, at the surface of the liquid, there are fewer neighboring molecules for the surface molecules to bond with. Thus they bond with each other to form an imaginary shell acting like a thin sheet of rubber around the drop. This outside surface acts as a force to keep the drop spherical and is described as surface tension. Surface tension is a property of a liquid.
Contact angle is used to measure the relative strength of these competing forces. As surface tension increases, the contact angle will increase as the drop tries to bead up. As the surface energy of the solid increases, the contact angle will decrease as the drop tries to wet and spread out to satisfy as many of those unsatisfied bonds on the solid's surface as possible. Young's equation (shown in first graphic above) illustrates how contact angle is a function of these competing forces: solid surface energy and liquid surface tension - or, in other words, the work of adhesion and the work of cohesion as illustrated in the graphic below. I should point out that there is an additional force referred to as solid/liquid interfacial free energy. This force is the result of the interaction between the liquid and the solid at the solid/liquid interface.
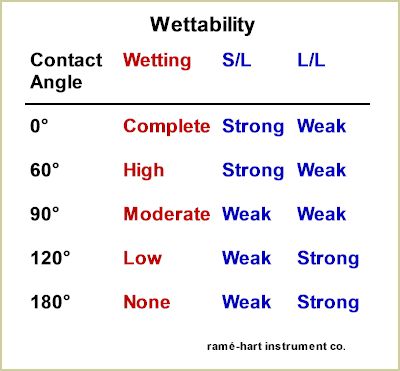
The chart below shows how contact angle not only characterizes wetting but is used to explain the relative strength of the competing adhesive forces at the solid/liquid (S/L) interface and the cohesive surface tension forces within the liquid (L/L).
Both surface energy and surface tension are force measurements and are measured in mN/m. Note that 1 mN/m is equal to 1 dyne/cm. The dyne/cm unit of measure is somewhat archaic and not commonly used today except in the printing and ink industry where it remains prevalent. Based on the above description of surface energy, surface tension, and contact angle, we conclude with the following summary points: 1. Contact angle is a function of the surface energy of the solid, the interfacial free energy at the solid/liquid interface and surface tension of the liquid itself. 2. If you know the surface tension of a test liquid (by either measuring it or by using a common liquid such as water with a published surface tension), you can calculate the surface energy of a solid by measuring the contact angle of test liquid on the solid surface. (Our DROPimage software does this automatically.) 3. A low contact angle indicates good adhesion, good wetting, and high surface energy and for many applications is desirable. 4. Contamination of the solid surface can lower surface energy resulting in poor adhesion and poor wetting and can be determined by higher measured contact angles. 5. There are many ways surfaces can be cleaned and treated to increase surface energy in order to improve adhesion and bonding. 6. Water has a surface tension of 72.8 mN/m at room temperature. Most liquids have a lower surface tension of between 20 and 50 mN/m. Most solids exhibit a surface energy under 100 mN/m but rarely under 10 mN/m. Most common polymers have a surface energy between 20 and 50 mN/m. 7. Environmental conditions, such as temperature and humidity, can affect surface tension and, to a lesser extent, surface energy. Surface roughness and homogeneity also affect contact angle. 8. Contact angle can be easily measured without an understanding of Young's equation, surface energy, surface tension, interfacial energy, or the myriad formulae relating thereto. 9. A contact angle goniometer measures contact angle and in some cases surface energy too. A goniometer / tensiometer can measure contact angle, surface energy, and surface tension. 10. Since 1961 ramé-hart has been the world leader in contact angle goniometry and offers a wide spectrum of goniometers and tensiometers designed to precisely and accurately measure contact angle, surface energy, and surface tension. If you have any comments or questions on the above article, or if you are in need of a contact angle goniometer, don't hesitate to contact us for pricing and more information. |
||
|
||