|
December
2025 |
| Why Measure the Contact Angle of MOFs? |
|
Recently, the Nobel Prize in Chemistry was
awarded to Susumu Kitagawa, Richard Robson, and Omar M. Yaghi for their
work in developing metal-organic frameworks, also known as MOFs.1
Their collective work, spanning over twenty years, brought this advanced
material to the forefront of the minds of many researchers. Since then,
MOFs have emerged as an exciting topic of study in the academic world
with some papers on the topic being cited tens of thousands of times.2
Industrial applications for MOFs, which are numerous, are expected to
reach revenues of hundreds of millions of dollars in the next ten years.3
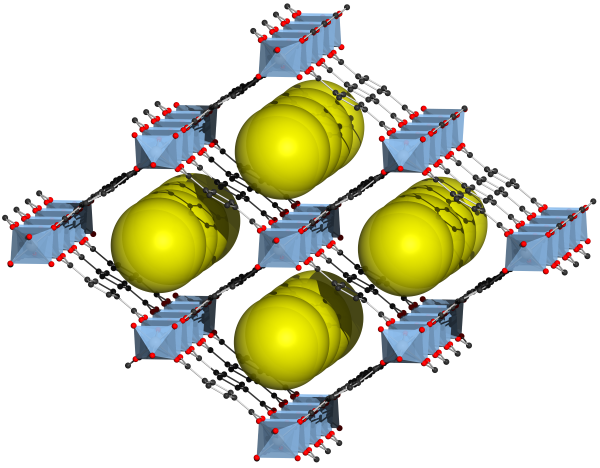
So, you might be asking, what are MOFs? The name "metal-organic framework" makes it easy to understand the molecular structure of MOFs. A metal complex is bonded to an organic molecule, typically a ligand, explaining the "metal-organic" part. This structure is then formed into an extended network, usually crystalline, hence the term "framework". The utility of the MOF is derived from this framework forming cavities or pores that are distributed regularly throughout the material. Molecules from outside the framework can then be captured in these pores for purposes such as extraction, storage or catalysis. Thousands of MOF compositions have been created and investigated around the world. Today, MOFs are useful in many different applications. One major application which MOFs are uniquely suited for is the manipulation of gases, including capture of carbon dioxide or moisture, and separating pollutants from air. Another promising area of research is using MOFs to catalyze reactions, causing captured molecules to break down into base components. MOFs can also be employed for water treatment, energy storage, drug delivery, sensing and biosensing, and many other processes that are part of our modern world. Researchers are coming up with new uses for MOFs all the time.
Each potential application for MOFs presents unique challenges. One common threat to the stability of a MOF is water. As an ideal ligand, water can compete with the organic component for bonding with the metal complex. Due to their capacious structures, MOFs tend to be structurally fragile. Once a few bonds have been broken or strained, the MOF can fall apart, possibly releasing whatever was captured in its pores. Some MOFs can avoid this issue by ensuring that water is not present, but many applications necessarily involve water, such as moisture capture or separating pollutants from water. Due to this problem, many recent studies concern themselves with the creation of MOFs that are hydrophobic and ways to synthesize hydrophobic MOFs.4 Hydrophobicity can be obtained through the formulation of the MOF or through post-processing. Investigating the hydrophobicity of any material begins with measuring the water contact angle. A surface can be characterized as being hydrophobic if the contact angle exceeds 90°. The goniometer is the ideal tool for performing contact angle measurements in a manner that is precise and repeatable. ramé-hart instruments have been used for contact angle measurements to characterize many MOF formulations.5,6,7 Hydrophobic metal organic frameworks are poised to play an instrumental role in diverse industrial and commercial applications. If you work with MOFs, contact us and let us help you find the right instrument for your application and budget. Notes |
| Measuring Advancing and Receding Contact Angle on a Curved Surface |
|
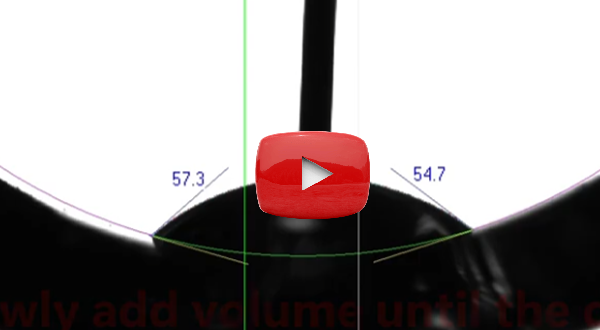
Our
latest video
demonstrates the powerful combination of the curved baseline feature in
the current version of DROPimage Advanced software coupled with the add/remove volume method
for precise measurement of advancing and receding contact angles.
This methodology offers an exceptionally robust way to determine contact angle hysteresis even on challenging, non-flat samples. This advanced capability solidifies ramé-hart's position as a world leader in developing powerful and versatile instrumentation for cutting-edge wetting analysis and contact angle determination. |
|
Regards,
Carl Clegg |